Effect of environmental toxins on GATC methylation in E. coli May 3, 2011
Posted by ljsteele in Biology, Chemistry, Ecology, Environment/Conservation, Evolution, Genetics, Health, Marian University curriculum, Physiology.1 comment so far
With the end of the semester drawing near it is becoming that time again when the results are piling in from research you have been working on all semester. As we speak, the final data collection and analysis is taking place in biochemistry, a team of student researchers are exploring of environmental toxins of DNA methylation in the bacterium E. coli. 
The Bacterial Genome
Bacteria exist throughout the world and can survive in almost any climate . Bacteria are unicellular and can consist in a wide range of environments such as a pond all the way to soil. One unique attribute of the bacterial genome is that it contains adenine methylation , opposed to mammalian organisms which contain cytosine methylation at GpC islands. Adenine methylation is when a methyl group becomes attached to the adenine nucleotide on the DNA. When a methyl group is donated from SAM to form a covalent attachment, it is made on the adenine which can cause steric hindrance of transcription factors and differential effects of DNA binding proteins, which can contribute to a change in gene expression. In previous studies it has been shown when E. coli is exposed to different carbon sources (ie glycerol or glucose). Some areas of the genome become demethylated. In the bacteria E. coli almost every adenine (A) in the GATC sequence is methylated. To block the methyation at the GATC sequence, a protein must be present to inhibit the DAM methyltransferase from depositing a methyl group on the adenine.
What does Methylation do?
Adenine methylation has many roles in bacteria. Methylation can effect gene expression, cell cycle, virulence, and how proteins interact with the DNA. For the research we are performing, we are concerned with what effect the environment has on changing adenine methylation on the GATC repeats. There are about 20,000 GATC repeats in the E. coli genome and under normal log growth conditions almost every single repeat is methylated. It has been found that when bacterial cells are in a log growth phase there are 6-10 sites which are not methylated. These nonmethylated sites lie up and down stream of promoters of different genes. The lack of methylation may allow DNA binding proteins to modulate their function to allow a functional change in gene expression.
Pollutants and the Genome
In the study we are performing we wanted to see how three classes of chemicals pollutants commonly found in the Midwest affect adenine methylation at the GATC site. We choose three pollutants to represent chemicals that fit into the families of common water pollutants, which are heavy metals, chlorinated compounds and nitrogen rich compounds.
The above families of compounds will be compared to samples collected from different areas around the campus of Marian University, Indianapolis, IN. Supplements will be added to all the samples to generate a rich liquid media that will facilitate bacterial growth. With 6 different test groups and 2 controls we are going to seek to determine if any of our known compounds or a compound present in our environmental sample has an effect on the methylation. The determination of methylation can be done by using restriction enzyme digest with endonuclease selecting specifically for the nonmethylated site. The enzyme we have chosen was MBO and AVI. When all the genomic DNA from the bacteria is extracted and digested, then it will be ran on a gel to be imaged to determine if the bands of digested DNA differ depending on the chemicals present during growth. This is a time efficient way to examine if any changes in methylation levels have occurred.
What Does It All Mean?
For conclusion, the relevance of this study includes a few things. This study will provide evidence to show if environmental toxins have an effect on bacterial DAM methylation. One role bacteria play in an ecosystem is influencing the flow of nutrients which support plant and algae growth. The results of our proposed study may display that toxins have an effect on methylation patterns which could lead to an increase the mutation rate of the bacteria genome itself. Destructive mutations may decrease bacterial populations leading to a disruption in the ecosystems nutrient flow, hence disruptions in plant and algae growth with effect additional aquatic and terrestrial organisms.
Mystical “Catnip” May 3, 2011
Posted by mhostetler099 in Behavior, Biology, Chemistry, Health, Physiology.add a comment
So what is it about “catnip” that makes cats crazy, mosquitoes fly away and at the same time has seemingly no effect on human beings? In actuality, the better question is what are the distinguishing factors allow different organisms to interpret “catnip’s” chemical signal differently or not at all.
The 6th Sense (the vomeronasal organ in cats)
It is well documented that all mammals posses 5 senses (sight, taste, touch, smell, hearing); BUT could mammals have a 6th sense?! Some scientists would say YES and pinpoint this sense to be related to the mysterious vomeronasal organ located above the roof of the mouth. This sensory organ is attributed to sensing chemical signals from other organisms and the environment known as pheromones. The vomeronasal organ is present in most mammals and is considered a chemoreceptor organ which exists as a separate entity than the nasal cavity. Chemoreceptors detect chemical signals from the organism’s environment and transduce a physiological response accordingly. Studies indicate that nepetalactone (the chemical produced by “catnip”) is responsible for eliciting a psychosexual response in cats by mimicking a sex pheromone and interacting with the feline vomeronasal organ. Although human beings and felines are both mammals, they react to the chemical in “catnip” much differently than one another. “Catnip” elicits no response in human beings and a rather strong response in felines. The distinction between these responses can most likely be attributed to a physiological difference in the feline and human sensory system.
The Vomeronasal Organ in Humans
 The function of the vomeronasal organ in human beings is actually quite controversial. Studies on human embryos have indicated that the vomeronasal organ does correspond to the vomeronasal organ in cats and other mammals. Although the vomeronasal organ is common in both feline and human species, the organ in humans was thought by scientist to be vestigial (or no longer functioning). The vestigality of the vomeronasal organ in human beings may explain why humans do not react to chemicals in “catnip” however this is an unlikely explanation because studies have shown human beings can react to pheromones. Another explanation to the differing reactions could potentially be attributed to the physiological differences in the organs themselves (show left).
The function of the vomeronasal organ in human beings is actually quite controversial. Studies on human embryos have indicated that the vomeronasal organ does correspond to the vomeronasal organ in cats and other mammals. Although the vomeronasal organ is common in both feline and human species, the organ in humans was thought by scientist to be vestigial (or no longer functioning). The vestigality of the vomeronasal organ in human beings may explain why humans do not react to chemicals in “catnip” however this is an unlikely explanation because studies have shown human beings can react to pheromones. Another explanation to the differing reactions could potentially be attributed to the physiological differences in the organs themselves (show left).
So Why are Mosquitoes Repelled?
So why are mosquitoes seemingly repelled by some essential oils extracted from different plants and herbs (including “catnip”)? This question is a little more difficult to answer directly because little is known about insect sensory system. Studies have shown that mosquitoes are more attracted to people with high concentrations of steroids and cholesterol on the surface of the skin. Mosquitoes are attracted and repelled by certain pheromones. More than likely, the chemical nepetalactone in “catnip” is able to mimic a pheromone that triggers a chemical signal causing the insect to become repelled (acting as an insecticide).
 It is truly amazing that the same chemical can signal different responses in different organisms. The responses to chemical signals in the organism’s environment are evolutionarily beneficial; whether it be to attract a mate or flee from impending danger. According to a news report conducted by NPR the CDC is working on natural repellant consisting of extract from cedar tree. This substance is completely environmentally friendly and actually acts as an insecticide. It is able to kill the mosquitoes by blocking receptors on their nerve cells (absent in human beings). Although the chemical found in “catnip” is not known to be an insecticide, the similarity between natural extracts (from “catnip” and cedar tree) may certainly explain insects natural repulsion from them.
It is truly amazing that the same chemical can signal different responses in different organisms. The responses to chemical signals in the organism’s environment are evolutionarily beneficial; whether it be to attract a mate or flee from impending danger. According to a news report conducted by NPR the CDC is working on natural repellant consisting of extract from cedar tree. This substance is completely environmentally friendly and actually acts as an insecticide. It is able to kill the mosquitoes by blocking receptors on their nerve cells (absent in human beings). Although the chemical found in “catnip” is not known to be an insecticide, the similarity between natural extracts (from “catnip” and cedar tree) may certainly explain insects natural repulsion from them.
An ‘Amazing Race’ of the Senses April 29, 2011
Posted by abueno526 in Biology, Fun, Physiology.add a comment
The last team to check in may be eliminated…
The Amazing Race is a reality tv show in which pairs of contestants race  around the world in a challenge of wits, strengths, and abilities to try to ultimately come in first place and win the coveted million dollar prize and of course, bragging rights. Throughout its 18 seasons in the United States, contestants have been put through a wide array of challenges, including participating in an acrobatic act, carrying furniture and grains across the city, and identifying a correct tune being played in a sea of pianos.
around the world in a challenge of wits, strengths, and abilities to try to ultimately come in first place and win the coveted million dollar prize and of course, bragging rights. Throughout its 18 seasons in the United States, contestants have been put through a wide array of challenges, including participating in an acrobatic act, carrying furniture and grains across the city, and identifying a correct tune being played in a sea of pianos.
Tea anyone?

In a specific episode this season, the contestants were required to drink a cup of papaya mango tea in a small shop in China. Later on that day, they had to pick out that same flavor of tea from a table of hundreds and hundreds of different cups of tea by recognizing the smell and taste. Although a daunting task, all of the teams successfully completed the challenge by identifying the tea. But, with so many scents and flavors on the table, how were they able to identify the correct cup?
Olfaction – Odorants
All things considered, humans have the ability to recognize and distinguish 7,000 to 10,000 different smells. But how is this possible? The first thing to consider is the human capability to detect odorants, which are typically small organic molecules with some amount of volatility so they can be carried in a vapor from to the nose. These small odorant molecules are actually detected by their shape, not from any other physical properties that they exhibit. This means that the different smells come from the way the molecule interacts 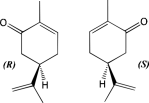 with the binding site it is associated with. A common example to better explain this idea can be seen in the molecule carvone (depicted left), which has distinct R and S configurations. Although the two are mirror images of one another, the R conformation has a scent of spearmint, while the S configuration of caraway, indicating their difference in binding.
with the binding site it is associated with. A common example to better explain this idea can be seen in the molecule carvone (depicted left), which has distinct R and S configurations. Although the two are mirror images of one another, the R conformation has a scent of spearmint, while the S configuration of caraway, indicating their difference in binding.
Olfaction – Odorant Receptors
Scents are detected in the main olfactory epithelium of the nose, and are are identified by one 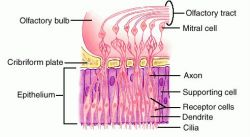 of the million sensory neurons that dwell there, which all contain cilia with receptors. Although we are able to recognize upward of 7,000 distinct scents, humans only have 350 odorant receptors As seen in the picture to the right,molecules bind to the receptors that are on the cilia, nerve impulses are generated from the binding and travel through the neurons, and finally move to the olfactory bulb. Throughout this process (binding to olfactory bulb response), cAMP and GTP levels in the body increase, meaning that the process uses 7TM receptors. These compounds are released in a cascade process, depicted to the left. When the odorant binds to the receptor, a G protein is activated and binds to
of the million sensory neurons that dwell there, which all contain cilia with receptors. Although we are able to recognize upward of 7,000 distinct scents, humans only have 350 odorant receptors As seen in the picture to the right,molecules bind to the receptors that are on the cilia, nerve impulses are generated from the binding and travel through the neurons, and finally move to the olfactory bulb. Throughout this process (binding to olfactory bulb response), cAMP and GTP levels in the body increase, meaning that the process uses 7TM receptors. These compounds are released in a cascade process, depicted to the left. When the odorant binds to the receptor, a G protein is activated and binds to
 GTP. This complex then moves to activate an adenylate cyclase, which increases cAMP levels. High cAMP levels activate and open ion channels, which creates an action potential and allows the smell of an odor to come through.
GTP. This complex then moves to activate an adenylate cyclase, which increases cAMP levels. High cAMP levels activate and open ion channels, which creates an action potential and allows the smell of an odor to come through.
Olfaction – Scent Recognition
But with only 350 distinct receptors, how are we able to detect thousands of smells? The answer lies in the fact that most smells are composed of several odorant receptors, which can be activated at different levels of odorant. In other words, there is not a one to one relationship for odorant to receptor, but instead odorants can activate multiple receptors and receptors can be activated by multiple odorants. As an example, the odorant C6COOH activates six different receptors, while C5OH, C6OH, and C7OH all activate the same receptor.
Olfaction gone awry
Sometimes, we are unable to detect some scents, called a specific anosmia. although everything seems to be functioning normally, certain compounds are not detected by these individuals, indicating that it it a genetic inheritance of a mutation. Although over 80 have been identified, some examples of molecules that are unable to be smelled include isobutyric acid, which is responsible for the smell of sweat, and n-Butyl mercaptan, the smell that skunks give off.
Gustation – An Overview
The tongue has the ability to recognize 5 major tastes in the mouth: bitter, sweet, salty,sour, and umami (savory). A diagram of where these individual taste buds are located can be found to the right, excluding the umami taste. “Umami” is a word derived from the Japanese language, and includes the tastes of glutamate and aspartate. Much less is known about this taste than the others because this “savory” flavor has only been distinguished from the others within
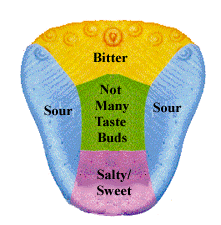
the past five to seven years. Receptors for tastants are more commonly referred to as taste buds, which are made up of about 150 cells. Microvilli on the surface of the tongue bind to tastants and send an impulse through the sensory neurons to the brain to identify the specific taste. The tastes use different methods to detect the taste, all of which are outlined below.
Gustation – Salty and Sour
Salty and sour tastes opperate in a similar manner in the fact that they both utilize ion channel interactions. In the case of salty flavors, this is done through sodium ion and their corresponding amiloride sensitive Na+ channels. Sodium ions pass through the channels on the front of the tongue creating a current, amiloride attempts to block this current, and a salty flavor can be tasted. Similarly, the sour taste acts through a hydrogen ion channel . Hydrogen ions flow through the pores on the sides of the tongue, and a sour taste is observed.
Gustation – Sweet and Bitter
Unlike the salty and sour tastes, both the sweet and bitter receptors utilize a 7TM receptor complex, as mentioned above in the olfaction discussion. Because of this, they respond to a larger range of stimulants. Sweet receptors typically respond to glucose, sucrose, aspartame, saccharine, and even some proteins. While being researched, scientists discovered that these compounds interact with the T1R1, T1R2, and T1R3 receptors in different combinations with one another. They all pick of variations of sweetness, with the T1R2 and T1R3 receptor being the most sensitive to the sugary taste and the T1R1 receptor by itself being the least sensitive to the taste. The bitter receptor acts in a similar manner, however, its receptors respond to toxic alkaloids. TR2 receptors are responsible for this taste, which is typically recognized at the back of the tongue. In this regard, it should be noted that taste receptors are much less selective than the scent receptors due to sheer number (350 vs. 5). For example, in the case of the bitter taste, we usually recognize just bitter in general and are unable to distinguish one bitter compound from another.
Gustation – Umami
The final taste is umami, which is recognized as the savory flavoring and utilizes 7TM receptors as well. These receptors respond to glutamete, aspartate, and even MSG. It is similar to the sweet receptor in the fact that it utilizes the T1R3 receptor, but it is also paired with the T1R1 receptor. Unlike the sweet receptor that may utilize different combinations of the receptor, the savory flavor can only be obtained with activation of both the T1R3 and T1R1 receptors simultaneously.
A Complimentary Combination
So, through a combination of the senses, contestants were able to identify the correct cup of tea. Using the odorant receptors to bind to the scent molecules and the specific taste buds on the tongue to identify the tastes, it is possible to identify a particular item in a sea of many. As a tip for the contestants for next time, they may want to rely on their nose more than taste due to the high specificity of the olfaction system!
April 5, 2011
Posted by Dr. O in Biology, Ecology, Environment/Conservation, Institute for Green & Sustainable Science (IGSS), Marian University curriculum, Physiology, Science Education.add a comment
Congrats to Marian University’s very own, Cassie Freestone! Check out her spread (click on picture to expand) in the Spring 2011 issue of Marian University’s magazine, The Magnet.

Cassie Freestone has participated in numerous independent research endeavors at Marian University from a rigorous summer research course at the Institute for Green & Sustainable Science, to taking independent research credits. Her research experience has given her the toolkit to attract and succeed in internship opportunities like this international marine research study.
Healing a “Broken” Heart March 4, 2011
Posted by Kyle in Biology, Chemistry, Health, Medicine, Physiology.add a comment
Irreparable Harm
The majority of those reading this have probably experienced some sort of injury in their lifetime. Injuries such as cuts and broken bones will soon heal with proper care, but there are certain tissues that if damaged, cannot repair themselves. Heart tissue and brain tissue are two examples that come to mind. This may be the case for most of us adult humans, but new research out of The University of Texas Southwestern Medical Center at Dallas is pointing out that some newborn mammals have the ability to heal completely when it comes to heart damage. The only problem is, at some point along the line, as we age, the heart loses this ability to heal itself. Still, this is a very important discovery for a society that suffers greatly from heart disease, which kills thousands of Americans every year.
 Studying a Broken Heart
Studying a Broken Heart
Researchers found that in newborn mice, when sections of heart were removed, the heart had completely healed within three weeks. The hearts then functioned as normal with no signs of damage. Understanding how this works and why the heart stops doing it after a certain amount of time is now the next step for researchers. Unlike when you tear a hamstring, damage to cardiac tissue after a heart attack doesn’t just heal with time. So for those who suffer from heart problems, a discovery like this brings them one step closer to a healthy heart in the future.
Of Mice & Men
Obviously mice, which help us a lot more than most people realize, and humans are a little different from each other, but seeing results like this in another mammal is still promising. If nothing else, it is definitely a huge step in the right direction for researchers looking to cut down on the number of heart related deaths. For now though, it is important for people to remember that they only have one heart, and taking care of it should be a priority.
Taking a Radioactive Drag: Polonium 210 and Cigarettes March 3, 2011
Posted by tsublett in Chemistry, Health, Medicine, Physiology, Policy.1 comment so far
The Unknown and Known Dangers of Smoking
Many of us know the dangers of smoking. We see many friends and loved ones diagnosed with cancer and know of many who die from it each year. We have seen the warning labels on cigarette packages, but what is actually in that smoke? Research says, it’s polonium-210, a radioactive isotope found in fertilizers. The problem is, tobacco companies knew about this, a while ago. According to a 2011 article in Scientific American, “The tobacco industry has known about polonium in cigarettes for nearly 50 years.” Facts like these are disconcerting on many levels.
Ways We Are Exposed to Polonium 210
How exactly this isotope gets into the tobacco leaf is not entirely known, but it is thought to be a “daughter isotope” of uranium 238 found in fertilizers. When the fertilizer is spread on the soil, it begins to decay into either an airborne isotope, such as radon 222, or into lead 210 in the soil. Both of these products enter through the roots or into the leaves and eventually decay into polonium 210. The leaves are then processed normally and eventually end up in cigarettes.
The History of Polonium 210 Detection
Now, then, there seems to be a problem. See, polonium 210 was detected first in the 1960s. This should be a BIG problem, because we are now considering its dangers even though it has been known about for 50+ years! Through a series of papers published during the 1960s, namely a paper published in 1964 by Radford and Hunt, scientists demonstrated how polonium 210 can enter the soil. Subsequently in a paper published in 1974, by John B. Little and William O’Toole, proved that smokers can develop “hot-spots” on their lungs where polonium 210 accumulates. The hot spots can cause mutations due to alpha decay . The problem is, tobacco farmers and cigarette manufacturers are not removing this isotope. The good news is… they may start doing so soon.
How much polonium do we get when we smoke?
Here is an excerpt from a New York Times article:
A fraction of a trillionth of a curie (a unit of radiation named for polonium’s discoverers, Marie and Pierre Curie) may not sound like much, but remember that we’re talking about a powerful radionuclide disgorging alpha particles — the most dangerous kind when it comes to lung cancer — at a much higher rate even than the plutonium used in the bomb dropped on Nagasaki. Polonium 210 has a half life of about 138 days, making it thousands of times more radioactive than the nuclear fuels used in early atomic bombs.
We should also recall that people smoke a lot of cigarettes — about 5.7 trillion worldwide every year, enough to make a continuous chain from the earth to the sun and back, with enough left over for a few side-trips to Mars. If .04 picocuries of polonium are inhaled with every cigarette, about a quarter of a curie of one of the world’s most radioactive poisons is inhaled along with the tar, nicotine and cyanide of all the world’s cigarettes smoked each year. Pack-and-a-half smokers are dosed to the tune of about 300 chest X-rays.
Is there any relief?
Maybe we should stop smoking, it’s likely the best approach. If you can’t quite kick the habit, the FDA may help. Recently the FDA has taken over the regulation of cigarettes in the wake of the Family Smoking and Tobacco Control Act passed in 2009. With the FDA’s help, the exact content of polonium 210 in cigarettes may soon be published. On a side note, one quick fix may come in tobacco leaf preparation. Simply washing the leaves after harvest may eliminate a large portion of the polonium 210 found in the air.
The Largest Preventable Cause of Death in the World.
It seems like a radioactive isotope found in smoke is just one of many carcinogens that continue to contribute to tobacco being the largest preventable cause of death in the world. According to Scientific American:
The World Heath Organization has made clear that smoking is the most avoidable cause of death. It estimates that 1.3 million people die of lung cancer worldwide every year, 90 percent because of smoking. If polonium has been reduced through methods known to the industry, many thousands of those deaths could have been avoided. The industry, many thousands of those deaths could have been avoided. The industry’s lawyers made the conscious choice not to act on the results of their own scientists’ investigations. But it is the customers who have had to live with-and die from- that decision.
So, cigarettes are bad, but how bad they may be for us is still up in the air. Perhaps we can make them a little less dangerous in the future by removing these dangerous isotopes. Hopefully, with the FDA regulating cigarettes, this dangerous vice will soon be put to rest.
Mysterious Melatonin December 18, 2010
Posted by Kyle in Biology, Chemistry, Health, Medicine, Neuroscience, Nutrition, Physiology.3 comments
I am sure everyone has already heard of a little compound known as melatonin. Melatonin is a hormone that can be found in many different organisms including plants, although most people know melatonin for its actions in mammals. In humans, melatonin is produced in the brain by the pineal gland. Circulating melatonin levels have been found to be high at night and low during the day, which is consistent with research that has shown that light suppresses melatonin. Because melatonin plays a role in controlling the circadian rhythm, it has received much interest for its use as a treatment for various sleep disorders. Because melatonin is a hormone, supplementing melatonin can present some issues.
Many people have used melatonin supplements to help them sleep at night. If you take a trip to your local drug store you are likely to find melatonin on the shelf. The first time I came across melatonin supplements I couldn’t help but think about the potential negative aspects to selling melatonin over the counter, unregulated. As many of you know, the human body likes to maintain homeostasis. When this delicate balance is interrupted, the body will react to return to homeostasis. I started to wonder what happens when someone takes melatonin supplements. The first thing that comes to mind is a decrease in the amount of melatonin receptors or a decrease in the production of melatonin itself, or both. I also wondered about possible side effects of increasing melatonin levels. As we have seen with many other hormones, multiple pathways and mechanisms can be influenced by a single hormone. So someone taking melatonin to help them sleep could inadvertently throw off other pathways, like those involved in reproduction for example.

Melatonin has been shown protect against reactive oxygen species, which can wreak havoc inside cells. This could potentially be an obvious benefit to taking melatonin supplements, especially if it helps an individual sleep at night. While sifting through the literature, I was unable to find any studies specifically looking at the negative effects of taking melatonin supplements, if any. But just because it isn’t proven that something is bad, doesn’t mean the potential for bad isn’t there. Also, other countries have taken action to stop over the counter sale of melatonin. Of course, there is also the question, do melatonin supplements even work? How much of the melatonin present in a melatonin pill is denatured by stomach acids or excreted in urine before it even has an effect?
I am skeptical of melatonin supplements, if you haven’t noticed yet. To each his own, but I don’t think I will be purchasing or taking any melatonin supplements in the near future. Good luck to everyone on their upcoming finals. Make sure to get plenty of sleep, although if your to-do list looks like mine, that won’t be happening.
Dang My Appetite! December 8, 2010
Posted by wframe488 in Behavior, Biology, Health, Medicine, Nutrition, Physiology.add a comment

The Biggest Loser
It wasn’t until recently America realized just how overweight people were getting in our country. I believe we are one of the most overweight countries of the world if I am not mistaken. It seems like new diet plans, weight-loss pills, and surgeries are developed everyday to help obese Americans shed those pounds. Weight-loss has definitely been growing its popularity, for example new reality television shows like The Biggest Loser , Weighing In, and Celebrity Fit Club, to name a few, have gotten people interested in getting up off the couch and exercising.
The fast food industry and video games can be partially blamed for helping Americans achieve the great honor of being one of the “biggest” countries in the world, but let’s not forget about our genetics. There are several hormones in our endocrine system that plays a role in weight regulation and weight-related behaviors like hunger and satiation. Two of the most popular and most talked about weight regulating hormones would have to be ghrelin and leptin. We all typically produce these hormones, but in different amounts depending on the person. Ghrelin is a preprohormone that is normally produced in the stomach. It is a known appetite inducer and has also been known to slow down metabolism and decrease the body’s ability to burn fat. It stimulates the hypothalamus to release growth hormone via a GSH receptor. Leptin, on the other hand, is known to aid in appetite inhibition. It is expressed predominantly by adipocytes and contains highly expressed receptors in the hypothalamus region of the brain. It stimulates the hypothalamus via an Ob receptor to decrease appetite and body weight.

Ghrelin and Leptin Action Summary
One research article that I found about this particular topic, by J.P.H. Wilding, was titled “Food Fails to Suppress Ghrelin Levels in Obese Humans”. This research paper investigated the effects of a test meal on the plasma levels of both ghrelin and leptin. They sampled 13 lean and 10 obese subjects and found that the lean subjects exhibited a decrease in both ghrelin and leptin levels after a meal whereas the obese did not show any signs of decrease in concentration of these two hormones. The paper goes on to explain that the role of the decline in leptin of the lean subjects is unknown, but the lack of suppression following a meal of the obese subjects could lead to increased food consumption. This suggests that ghrelin is involved in the pathophysiology of obesity. This appetite inducing hormone is secreted by our bodies with out our control unfortunately and for those that secrete more will mostly likely tend to be bigger human beings just based off of overall caloric intake.

Food Groups
In regards to dieting, one major problem that almost all people possess after they diet is the regain of weight. This mainly is due to the idea that even though your weight is now maintained at a healthy level your appetite still remains the same as it once was, thanks to these two hormones mentioned previously. One interesting article that I read from the Journal of Clinical Endocrinology and Metabolism, was titled, “Appetite Hormones May Predict Weight Regain After Dieting”, which was by Ana B. Crujeiras. Her colleagues and her evaluated a group of 104 overweight men and women during an 8-week low-calorie diet and again 32 weeks after treatment. The scientists measured body weight and plasma ghrelin and leptin levels before, during, and after dieting. What is interesting about this article is that the researchers found that subjects with higher plasma leptin and lower ghrelin levels before dieting were the subjects that were more prone to regaining weight after they shed those pounds through dieting. Personally, I thought that the higher the ghrelin levels prior to dieting would cause the subject to be more prone to weight regain, but that’s not the case here, but that’s science. The article goes on to explain that this can be useful information and that these hormone levels can be proposed as biomarkers for predicting obesity-treatment outcomes.
In conclusion we know that virtually everyone produces ghrelin and leptin in there bodies and that these two hormones play a big role in regulating our appetite. Some of us are lucky enough to sustain the proper balance of these hormones, based solely on our genetics, for body weight maintenance. Although, others aren’t so lucky to possess such a talent. Just because someone is lean and skinny doesn’t mean that they are necessarily healthy, and just because someone totes around more body weight than others doesn’t mean that that person is necessarily unhealthy. In closing, all I have to say is that eating right and exercising is a big part of being healthy and maintaining weight despite what these pesky hormones are doing to our appetite. So, to everyone, eat healthy and exercise!
Don’t Get Tipsy Over Your Hormones October 21, 2010
Posted by jfalender232 in Behavior, Biology, Health, Physiology.add a comment
Okay here’s the scoop, instead of boring you with the likely event that my fellow classmates might have bestowed upon you with their own blog posts, luckily for you, I pledge to make this the most exciting blog post that you will have the opportunity to read (that means you SHOULD click on the words that are highlighted in blue and underlined–you can thank me later for all the joy these links will give you). So enough with the chit-chat for now, let’s get ready to rumbbleeeee!
College is all about experiencing many new things such as moving away from your annoying parent(s), skipping class because it might be raining outside, meeting a myriad of new people, learning the art of mooching off your friends, and finally being exposed to your new best friend but your worst enemy. No I am not talking about Dean Wormer, hopefully you will never have to meet the dean. I am talking about alcohol, booze, liquor. It could make you the most popular person at night but then keep you strapped down to the bed with the worst hangover imaginable (unless you have to deal with Mike Tyson). Please allow me to whet your educational appetite with this nugget of information: “Alcohol dilates the blood vessels, or capillaries, that carry blood just below the surface of the skin. When they expand, the flow of blood to the skin is increased. The skin flushes, causing a warm feeling.” Alright so now that we are all warm and fuzzy inside lets jump in and explore this topic some more.
Alcohol and has many effects on the human body but one of the most important areas of research is the relationship between alcohol and hormones. WedMD defines hormones as “a chemical substance, formed in one organ or part of the body and carried in the blood to another organ or part where they exert functional effects; depending on the specificity of their effects, hormones can alter the functional activity, and sometimes the structure, of just one organ or tissue or various numbers of them.” Furthermore, alcohol has 4 primary areas that can effect: the regulation of blood sugar levels, reproductive functions, calcium metabolism, and bone structure.
Contrary to Def Leppards wish, I don’t want you to pour some sugar on me, so by realizing that your alcohol drinking can affect all three of your glucose sources and the functions of regulatory hormones will go a long way towards having a healthy relationship with your body. “Even in well-nourished people, alcohol can disturb blood sugar levels. Acute alcohol consumption, especially in combination with sugar, augments insulin secretion and causes temporary hypoglycemia. In addition, studies in healthy subjects and insulin-dependent diabetics have shown that acute alcohol consumption can impair the hormonal response to hypoglycemia (*More Info*)”. Maintaining normal blood sugar levels are crucial to the homeostasis of your body.
I would like to take a time-out here and impart some of facts of college life as told by yours truly (WARNING: please take all these facts with a grain of salt): You will spend more time thinking about sex than anything you might learn in a class. That being said, alcohol can have some potentially serious side effects on the reproductive system of both males and females. “In men, reproductive hormones are responsible for sexual maturation, sperm development and thus fertility, and various aspects of male sexual behavior. In women, hormones promote the development of secondary sexual characteristics, such as breast development and distribution of body hair; regulate the menstrual cycle; and are necessary to maintain pregnancy. Chronic heavy drinking can interfere with all these functions. Its most severe consequences in both men and women include inadequate functioning of the testes and ovaries, resulting in hormonal deficiencies, sexual dysfunction, and infertility (*More Info*)”. This is important to keep in mind because for males, extended low levels of testosterone can lead to the developing of feminization of males characteristics such as “breast enlargement”. Women need to be aware of alcohol effects because prolonged drinking can lead to “cessation of menstruation, irregular menstrual cycles, and menstrual cycles without ovulation, early menopause, and increased risk of spontaneous abortions (*More Info*)”. This leads to a creed that all should take to heart: ‘You can always dump your boyfriend or girlfriend, but never ever dump your hormones.’
The final two areas that we are covering in-depth, calcium metabolism and bone structure are closely correlated to one another. Calcium is the main building block of bones and is essential to the cell to cell communication. Speaking of communication relationships, ‘America’s favorite life-guru’ Dr. Phil can answer any communication issues you might have here.
The role of alcohol in calcium and bone metabolism can lead to several complications. “Acute alcohol consumption can lead to a transient parathyroid hormone (PTH) deficiency and increased urinary calcium excretion, resulting in loss of calcium from the body (*More Info*). Chronic heavy drinking can disturb vitamin D metabolism, resulting in inadequate absorption of dietary calcium (*More Info*)”. These decreased calcium levels can potentially lead to bone diseases, most notably, osteoporosis. MedicineNet describes osteoporosis as, “a condition characterized by a decrease in the density of bone, decreasing its strength and resulting in fragile bones. Osteoporosis literally leads to abnormally porous bone that is compressible, like a sponge. This disorder of the skeleton weakens the bone and results in frequent fractures in the bones”. So yes it is possible that if you are not careful, excessive alcohol consumption could lead you to looking like this dashing fellow below.
So the next time you and your friends start to pour shots, shotgun beers, or do keg stands to the greatest 80s song of all time Here I Go Again, just remember that your hormones are relying on you as their designated driver for the night.
Cheers!
Please check out my friend, Wes’ blog, who examines more about alcoholism and its effects on the endocrine. It’s a great read!
Decreasing Ageing affect on Memory October 15, 2010
Posted by zach in Health, Medicine, Neuroscience, Physiology.add a comment
Have you recently misplaced your car keys and spent hours trying to find them? A resent article from Science Daily explains how misplacing your keys may be a thing of the past. A promising new drug candidate is currently being developed at the University of Edinburgh to reverse age-related memory loss. The researchers have developed a compound that has improved cognitive function and memory in aging mice. This compound works by blocking an enzyme known as 11beta-HSD1. As we age our body changes, with these changes comes changes in the concentration of the enzymes in our body. The cause of these enzymatic changes is not fully known but it can be linked to physiological effects such as stress.
The aging enzyme
11beta-HSD1 is an enzyme that is found in the brain which can produce stress hormones such as the glucocorticoids. When there are high levels of glucocorticoids in the brain negatively affect memory. Therefore, if we can find a way to block 11beta-HSD1 we could increase our memory by decreasing the negative pressure on memory. The problem with blocking 11beta-HSD1 is that until now it hasn’t been possible to find a molecule that has a high specificity for blocking only 11beta-HSD1. After ingesting a synthetic compound that blocks 11beta-HSD1, mice show a dramatic increase in memory after only ten days. The increase in memory was quantified by the time it took mice to complete a Y maze.
A burgeoning field of research
The research in the biomedical world is very concentrated on developing medicines that will reduce or even try to eliminate the effects of aging. In the past I have blogged about how targets of rapamycin act as a master regulator for protein synthesis. If we could find a drug to regulate that regulated TOR we could in turn regulate aspects of how our body ages. Maybe some day we will have a set of anti-aging drugs that will allow us to combat all the negative effects that come with growing old. If researchers can keep developing synthetic compounds to stop memory loss there may be a day when you will never forget where you misplaced your keys.






